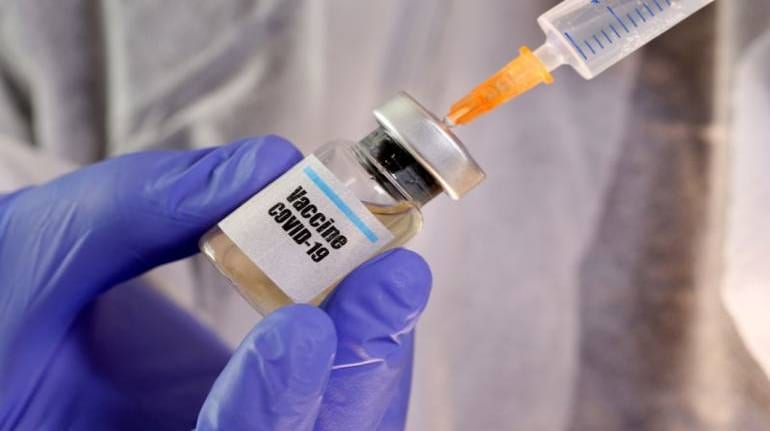
Companies leading the race to make the coronavirus vaccine can produce 70 to 75 million doses by the end of December. This would be sufficient to immunize about ten percent of the world's population.
The vaccine race includes American company Moderna, Johnson & Johnson, British company AstraZeneca, Gamelaya Research Institute of Russia, and Synovac of China. Pfizer, which is making the vaccine with the German company Biotech, also said on Friday that it could submit the results of the vaccine test to the regulatory agency for review by the end of October. If approved, by December she will prepare 100 million doses. Experts say that Pfizer has also become one of the fastest vaccine-producing companies by the October target. Pfizer has also signed a two billion dollar agreement with the US government to prepare 100 million doses for the US.
Johnson & Johnson's final test from September
Phase III trials of the US forma company Johnson & Johnson's vaccine will begin in September. The company will conduct vaccine trials in 180 places at 60 thousand people above 18 years of age. The company's chief scientific advisor, Dr. Paul, says that we will complete the test on a fixed timeframe.
Russia will start 40 thousand trials
Russia's Gamalaya Research Institute will conduct Phase III trials of the Sputnik V vaccine at 40 thousand volunteers. However, it has already decided to produce the vaccine from September. Vaccination will start in the general public from January onwards after health workers in the initial phase. Dr. Dennis Logunov, deputy director of the institute, said that this would allow us to assess the vaccine's immunity.
Operation Warp Speed in America
Under the Operation Warp Speed, the US government has tied up with more than six companies to get around 1.5 billion doses of the vaccine. It has spent $ 11 billion on it. These include AstraZeneca, Moderna, J&J.
Where many vaccines will be made by the end of the year ---
Pfizer Biotech to produce 100 million vaccines by year-end
30 million vaccine serum can be prepared by December
Moderna will prepare 10 to 15 crore vaccines by the end of the year
50 million to 10 million vaccines per month. Gamelaya Institute of Russia
China's cancino to make 01 to 03 crore doses per month
Synovac to produce 30 million doses per month by December
Conflict over-vaccination before the election in America
There has been an uproar over President Donald Trump's campaign to prepare the vaccine ahead of the November 4 election in the US. Food and Drug Administration (FDA) officials approving the vaccine have said they would resign if the vaccine was given a rubber-stamp-like vaccine without assessing its efficacy.
Trump himself has said that the vaccine may come before the election on November 3. Peter Marks, director of the FDA's Center of Biologics Evaluation and Research, openly threatened this on Friday. Last week, several government officials, pharmaceutical companies CEOs and medical experts had expressed concern that the vaccine was expected to be approved by the US, like Russia, by flouting standards. They are all included in the vaccine workgroup of the National Institutes of Health, which is responsible for making vaccines.
Experts have feared that the Trump administration is pressuring the FDA to approve a rough vaccine before November, whether clinical trials are completed or not, for the benefit of the election. Marx said that if unsafe and ineffective vaccines were introduced without scientific tests, he would not delay even a moment in leaving the post. Assistant Secretary in the Department of Health Michael Caputo also said that political pressure on the vaccine will reduce the importance of FDA, which is reputed in the world.
Volunteer only got ten percent of the claim on damages
Volunteers whose health was damaged in vaccine testing in the US were paid only ten percent of the claim amount. This has been revealed by the CICP, the harm monitoring body for vaccine human trials under the US Department of Health. Volunteers suffering physical side effects from the ongoing 16 vaccine trials in the US had sought this compensation from the government. However, it is not clear whether the corona vaccine is involved.
China surrounded by giving vaccine without approval
A Chinese mining company in Papua New Guinea claimed that testing of a vaccine for Covid had developed immunity among its employees. Following the revelation, the country's Health Minister Papua Zelta Wong said that his department is investigating the claims of the Ramu Niko Management Company. He said that there is a ban on the testing of Covid-19 vaccines in the country. An answer will be sought from China in this regard. 48 Chinese employees have been given the coronavirus vaccine on August 10.
Vivek Agnihotri starrer 'Bengal Files' is currently surrounded by controversies. There was a lot
BAN vs IRE: Mushfiqur Rahim completes century on the last ball, records against Ireland
Veteran Bangladesh batsman Mushfiqur Rahim created history by scoring a century in the second ODI
Atlas Air's Dreamlifter, the main landing gear tire of a Boeing 747 aircraft detached from the ai
On March 19, the death anniversary of the late Punjabi singer Sidhu Moosewala was organized in Ma
Twitter boss Elon Musk announced his resignation as CEO of the company on Wednesday. He tweeted &
Today is the birthday of fans' favorite Bhaijaan i.e. Salman Khan. On his birthday, he is getting
Concerned about rising inflation, the Reserve Bank of India has increased the repo rate by 0.50%.
The date of debate has been approved in the country's highest court against another rule of 'Mu
Congress leader Priyanka Gandhi Vadra has again become corona infected. He has given this informa
Adani Group's 74% stake in Mumbai Airport, agreement for the acquisition
The Adani Group will acquire GVK Group's stake in Mumbai Airport. The group, led by billionaire i